MR pulse sequence development, electromagnetic transducer design, reconstruction algorithm development, image registration and analysis
PhD candidate Ramin S. Sahebjavaher (co-supervised by S.E. Salcudean and R. Sinkus), Post-doctoral fellow Ali Baghani (co-supervised by S.E. Salcudean and R. Rohling), PhD candidate Guy Nir (supervised by S.E. Salcudean), and PhD Candidate Mohammad Honarvar (co-supervised by S.E. Salcudean and R. Rohling).
Grants: Natural Sciences and Engineering Research Council of Canada (NSERC), Canadian Institute of Health Research (CIHR), BC Innovation Council NRAS Program, and the Prostate Cancer Foundation BC (PCFBC).
In MR elastography (MRE), the shear modulus (i.e., stiffness) is obtained by inverting the waves equation based on the measured wave field in the object of interest [1]. The wave field is acquired using a motion sensitive sequence that is synchronized to the vibrations generated by an external transducer.
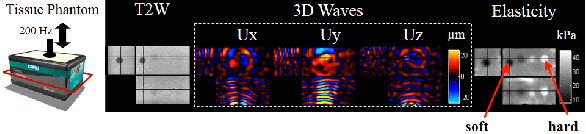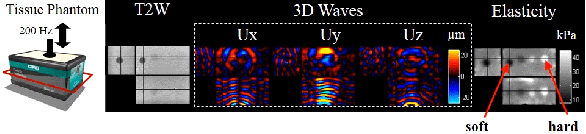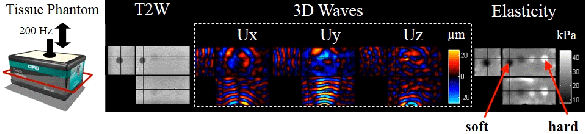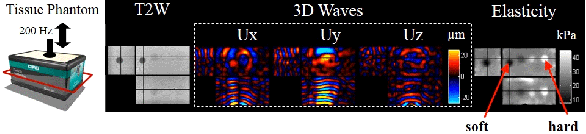
A T2-weighted image and the 3D wave field (200Hz) of a phantom with inclusions of various stiffness. The inclusions can be easily visualized in the reconstruction elastogram.
Intrinsic mechanical properties (such as the shear modulus) correlate well with tissue abnormalities in a number of applications in the field of elastography [2], [3], [4], [5], [6]. In prostate cancer elastography, ultrasound is leading the field due to its wide availability and being real-time [7], [8], [9]. An advantage to MRE over ultrasound elastography methods is that it can acquire 3D wave field (as opposed to 1D or 2D displacement tracking by ultrasound) which enables the full inversion of the wave equation thereby improving accuracy.
We are developing:
• Magnetic Resonance Elastography systems for 3T Philips (in-vivo) and 7T Bruker (ex-vivo) scanners for prostate cancer imaging
• MR compatible transducers for prostate MR elastography
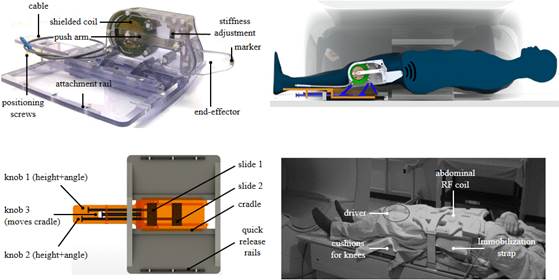
MR compatible shielded electromagnetic transducer for Trans-perineal MRE
• MR pulse sequences for rapid imaging of the 3D displacement field for clinical use
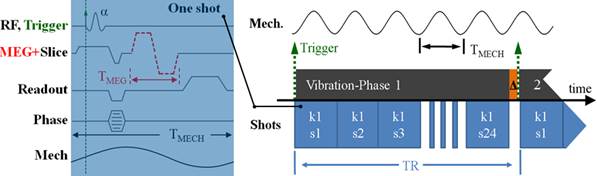
Fractionally encoded multi-slice steady-state gradient echo pulse sequence (eXpreeso) for imaging of prostate and liver
• Approaches to the inverse problem based on direct inversion and finite elements methods
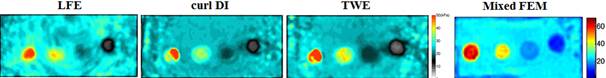
Reconstruction result based on Local frequency estimate, curl-based direct inversion, traveling wave expansion and mixed finite-element-method.
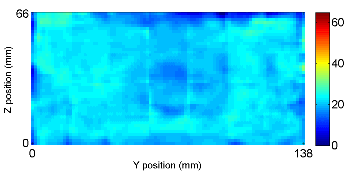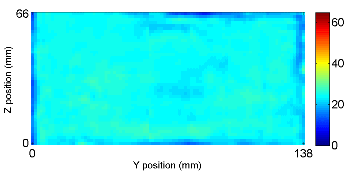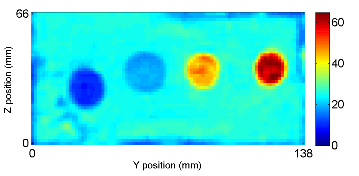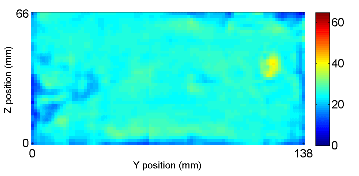
3D reconstruction result using mixed FEM with sparsity regularization
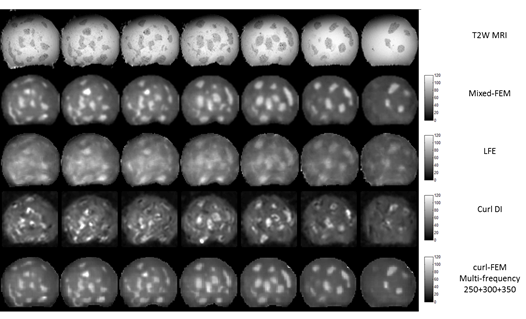
Reconstruction results of agar phantom with irregular hard inclusions using different methods
• Semi-automatic image segmentation and multi-modality registration based on shear modulus
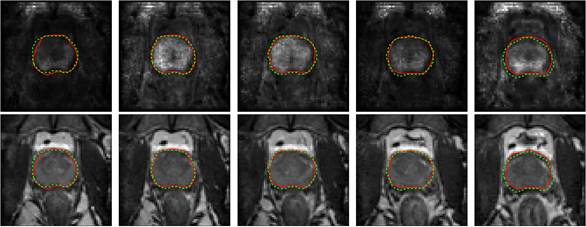
Prostate segmentation based on MRE elasticity and magnitude images

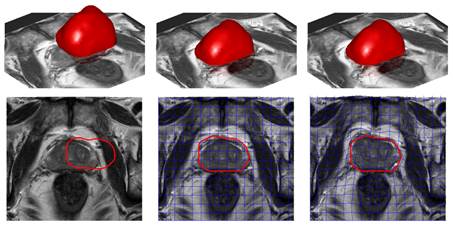
Model-based registration of an ex vivo model to in vivo MRI of the prostate, regulated by elasticity information acquired by MRE

Slice-to-volume registration of histological slices to MRI and MRE of the prostate using particle filtering
The clinical evaluation of the methods is currently being evaluated in a prostate cancer patient study.
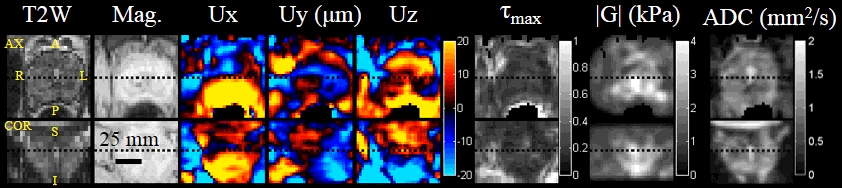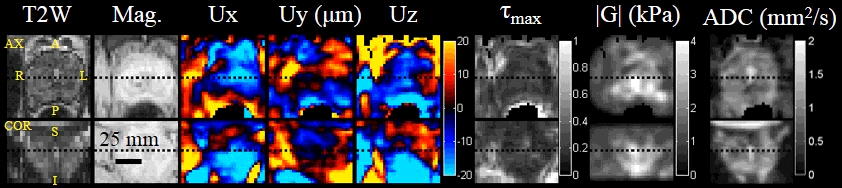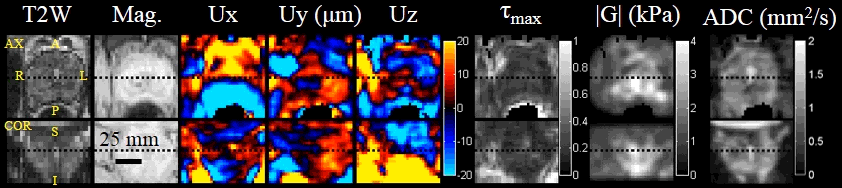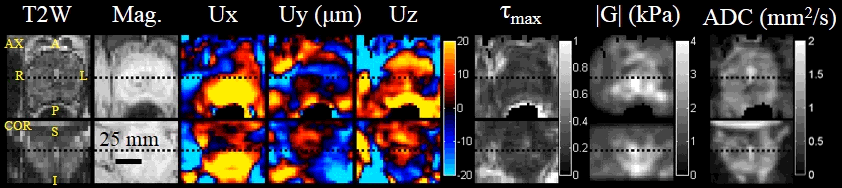
Sample images for the patient study. Images shown in axial and coronal projections from left to right: T2-weighted, magnitude image of MRE, 3D waves, reconstructed shear modulus |G|, max shear strain τmax, and ADC.
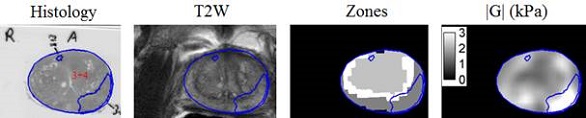
T2-weighted, prostate zones, and shear moduli |G| as registered to histopathology slides. The large cancer tumor on the right side of the image can be distinguished with ease in the MRE image |G| but not in the traditional T2-weighted MR image.
See papers with Sahebjavaher, Baghani, Nir and Honarvar for recent work.
• Sahebjavaher RS, Baghani A, Honarvar M, Sinkus R, Salcudean SE, Trans-perineal Prostate MR Elastography: initial in-vivo results, Magnetic Resonance in Medicine, vol. 69, no. 2, pp. 411–20, 2013.
• Sahebjavaher RS, Bylinskii A, terBeek L, Garteiser P, Sinkus R, Salcudean S, Rapid 3D MR Elastography of the Prostate with a Shielded Electromagnetic Transducer and Multi-slice Steady-state Gradient Echo Sequence: design and characterization results, Journal of Magnetic Resonance Imaging, submitted. (submitted to JMRI)
• Baghani A, Salcudean SE, Honarvar M, Sahebjavaher RS, Rohling R, Sinkus R, Travelling Wave Expansion: a model fitting approach to the inverse problem of elasticity reconstruction, Medical Imaging, IEEE Transactions on 30, no. 8 (2011): 1555-1565.
• Honarvar M, Sahebjavaher RS, Salcudean SE, Rohling R, Sparsity Regularization in Dynamic Elastography, Physics in Medicine and Biology 57, no. 19 (2012): 5909.
• Garteiser P, Sahebjavaher RS, terBeek LC, Salcudean SE, Vilgrain V, vanBeers B, Sinkus R, eXpresso: a novel gradient echo sequence for magnetic resonance elastography, NMR in Biomedicine 2013. (in press, NBM-12-0238)
• Honarvar M, Sahebjavaher RS, Sinkus R, Rohling R, Salcudean SE, Curl-based Finite Element Reconstruction of the Shear Modulus Without Assuming Local Homogeneity: Time Harmonic Case, Transactions in Medical Imaging, 2013. (Submitted to TMI)
• Nir G, Sahebjavaher RS, Kozlowski P, Chang SD, Sinkus R, Goldenberg L,Salcudean SE, Model-Based Registration of Ex-vivo and In-vivo MRI of the Prostate Using Elastography, Transactions in Medical Imaging, 2012. (in press)
References
[1]R. Muthupillai, D. J. Lomas, P. J. Rossman, J. F. Greenleaf, A. Manduca, and R. L. Ehman, “Magnetic resonance elastography by direct visualization of propagating acoustic strain waves,” Science, vol. 269, no. 5232, p. 1854, 1995.
[2]J. Ophir, S. K. Alam, B. S. Garra, F. Kallel, E. E. Konofagou, T. Krouskop, C. R. Merritt, R. Righetti, R. Souchon, and S. Srinivasan, “Elastography: imaging the elastic properties of soft tissues with ultrasound,” J. Med. Ultrason., vol. 29, no. 4, pp. 155–171, 2002.
[3]M. Yin, J. A. Talwalkar, K. J. Glaser, A. Manduca, R. C. Grimm, P. J. Rossman, J. L. Fidler, and R. L. Ehman, “Assessment of hepatic fibrosis with magnetic resonance elastography,” Clin. Gastroenterol. Hepatol. Off. Clin. Pr. J. Am. Gastroenterol. Assoc., vol. 5, no. 10, pp. 1207–1213.e2, Oct. 2007.
[4]R. Sinkus, M. Tanter, T. Xydeas, S. Catheline, J. Bercoff, and M. Fink, “Viscoelastic shear properties of in vivo breast lesions measured by MR elastography,” Magn. Reson. Imaging, vol. 23, no. 2, pp. 159–165, 2005.
[5]I. Sack, B. Beierbach, U. Hamhaber, D. Klatt, and J. Braun, “Non-invasive measurement of brain viscoelasticity using magnetic resonance elastography,” Nmr Biomed., vol. 21, no. 3, pp. 265–271, 2008.
[6]S. A. Kruse, G. H. Rose, K. J. Glaser, A. Manduca, J. P. Felmlee, C. R. Jack Jr, and R. L. Ehman, “Magnetic resonance elastography of the brain,” Neuroimage, vol. 39, no. 1, pp. 231–237, 2008.
[7]D. L. Cochlin, R. H. Ganatra, and D. F. R. Griffiths, “Elastography in the detection of prostatic cancer,” Clin. Radiol., vol. 57, no. 11, pp. 1014–1020, 2002.
[8]G. Salomon, J. Köllerman, I. Thederan, F. K. Chun, L. Budäus, T. Schlomm, H. Isbarn, H. Heinzer, H. Huland, and M. Graefen, “Evaluation of prostate cancer detection with ultrasound real-time elastography: a comparison with step section pathological analysis after radical prostatectomy,” Eur. Urol., vol. 54, no. 6, pp. 1354–1362, 2008.
[9]L. Curiel, R. Souchon, O. Rouviere, A. Gelet, and J. Y. Chapelon, “Elastography for the follow-up of high-intensity focused ultrasound prostate cancer treatment: initial comparison with MRI,” Ultrasound Med. Biol., vol. 31, no. 11, pp. 1461–1468, 2005.